Background: The combination of automation and expanding panel of target genes has improved utility and reduced costs of Next Generation Sequencing (NGS), leading to its widespread adoption in the clinical laboratory. In most laboratories, NGS has been added without consideration for redundancy or relative value compared to traditional genomic assays such as G-band karyotyping and FISH. At our centre, most patients with suspected hematologic malignancies receive both conventional cytogenetics (CG) and NGS assessment in addition to bone marrow morphology and flow cytometry. Appropriate test utilization is a high priority highlighted by campaigns such as Choosing Wisely, which often disproportionally focus on appropriate utilization of "routine" high volume tests rather than new test modalities. We implemented NGS screening in patients with suspected hematologic malignancies (Levi et al. 2019), and demonstrated enhanced diagnostic and prognostic yield of NGS, supporting the efficacy and cost-effectiveness of an 'NGS first' approach with CG restricted to samples with morphologic abnormalities in MDS (Kawata et al. BJH 2020). In this follow up study, we further expanded our Morphologic Flow Triage/NGS first (MFT-NGS1) algorithm to investigate patients with suspected hematological diseases. The main objective of our study was to evaluate this rationalized molecular diagnostic testing "MFT-NGS1" algorithm for its feasibility, acceptability and cost impact.
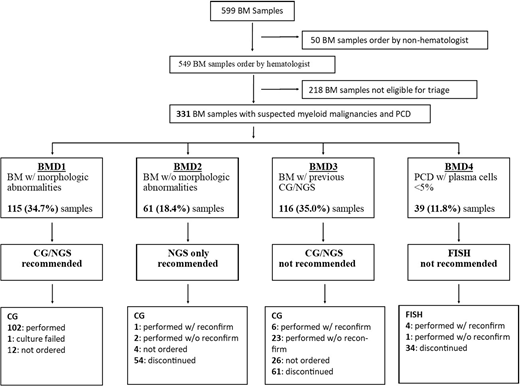
Methods: Using the results from morphologic interpretation of aspirate and flow cytometry, patient samples were triaged into 4 groups. Group 1: Patients with dysplastic features in the marrow or excess blasts were triaged to Bone Marrow Molecular Diagnostic 1 (BMD1) and had both NGS and G-band karyotyping. Group 2: Patients with no excess blasts or dysplasia (BMD2), had NGS only with CG sample held for 3 months in case testing was required in follow up. Group 3: Patients who had NGS and/or CG on a previous BM aspirate were triaged to (BMD3), with the comment that NGS and CG should not be repeated unless results will influence patient management. These samples were held for 4 weeks and testing was performed only if specifically requested. Group 4: Patients with suspected myeloma where the proportion of plasma cells was less than 5% were triaged to (BMD4), and FISH testing was cancelled. These samples were held for 3 months in case subsequent biopsy showed an increased proportion of plasma cells in keeping with myeloma.
Results: Over a 9-month period between August 2019 to April 2020 a total of 599 adult BM samples were assessed; 549 (91.7%) were ordered by hematologists and 331 (60.1%) meeting study criteria for MFT-NGS1 algorithm. Of those, 115 (34.7%) samples showed morphologic abnormalities and triaged to BMD1; 61 (18.4%) samples showed no morphologic abnormalities and were triaged to BMD2; 116 (35%) had previous CG and/or NGS tested and triaged to BMD3; and 39 (11.8%) samples were from patients with suspected myeloma had less than 5% of plasma cells and were triaged into BMD4 group. (Figure 1) Overall the MFT-NGS1 algorithm decreases G band karyotyping or FISH analysis in 149/331 (45%) samples. Only 11 / 216 (5.5%) hematologist overruled triage comment and requested CG testing for a specific indication either suspected progression of known diseases or for a therapeutic decision (e.g. drug approval). CG and FISH were mistakenly performed without the necessary reconfirmation in 26/216 (12%).
Conclusion: The proposed approach combining morphologic and flow triage and NGS as the primary genomic test (MFT-NGS) is both feasible and well accepted by clinical teams. We estimated that approximately 40% of all CG testing could be reduced primarily by reducing repeat testing which offsets a large proportion of the cost of implementation of NGS testing, while increasing both diagnostic and prognostic yield. Key factors for the success of this quality improvement project were involvement of clinical and genomic teams in developing the triage algorithm, rapid turnaround time (less than 24 to 48 hours) for aspirate interpretation and flow for triage and communication between laboratories within a fully integrated healthcare centre.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal